Lab members (right to left): Dr. Wood, Kaitlyn Malone, Alex Snider,
Katie Bidne, Andrea McCain, Kelsey Timme, Kerri Bochantin
 The overarching goal of the lab is to understand how chronic metabolic stress and inflammation impair fertility in female animals and women. We use multiple mouse models of obesity and a cow model of chronic ovarian inflammation to achieve this goal. Our current studies center on the impact of inflammation on oocyte quality which directly impacts successful fertilization and embryonic development with reduced oocyte quality significantly contributing to embryo loss. Recent studies also suggest that poor gamete quality alters the developmental trajectory of the embryo which in turn impacts subsequent placental and fetal growth and development. Thus, poor gamete quality may play a role in developmental programming of metabolic syndromes and abnormal reproductive function in viable offspring
The overarching goal of the lab is to understand how chronic metabolic stress and inflammation impair fertility in female animals and women. We use multiple mouse models of obesity and a cow model of chronic ovarian inflammation to achieve this goal. Our current studies center on the impact of inflammation on oocyte quality which directly impacts successful fertilization and embryonic development with reduced oocyte quality significantly contributing to embryo loss. Recent studies also suggest that poor gamete quality alters the developmental trajectory of the embryo which in turn impacts subsequent placental and fetal growth and development. Thus, poor gamete quality may play a role in developmental programming of metabolic syndromes and abnormal reproductive function in viable offspring
Research
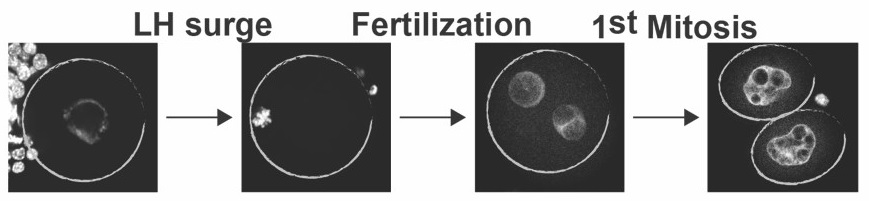 Female obesity increases the incidence of miscarriages and causes IVF failure, which has been attributed in large part to poor ooctyte quality. There is also overwhelming evidence that maternal (and paternal) obesity induces adaptive programming which predisposes the next generation of offspring to not only metabolic dysfuntion but also neurodevelopmental disorders and pediatric cancers. Likewise, metabolic disturbances in
Female obesity increases the incidence of miscarriages and causes IVF failure, which has been attributed in large part to poor ooctyte quality. There is also overwhelming evidence that maternal (and paternal) obesity induces adaptive programming which predisposes the next generation of offspring to not only metabolic dysfuntion but also neurodevelopmental disorders and pediatric cancers. Likewise, metabolic disturbances in  domestic livestock result in anovulation, early embryonic loss, and altered expression of important production traits in viable offspring including growth, meat quality, and reproductive metabolic disturbances not only affect her fertility but also the metabolic health of future generations. These studies could also lead to new management practices by livestock producers that increase food production and healthfulness for a growing world population.
domestic livestock result in anovulation, early embryonic loss, and altered expression of important production traits in viable offspring including growth, meat quality, and reproductive metabolic disturbances not only affect her fertility but also the metabolic health of future generations. These studies could also lead to new management practices by livestock producers that increase food production and healthfulness for a growing world population.
Oocyte
Effect of the Follicular Environment on Oocyte Quality: 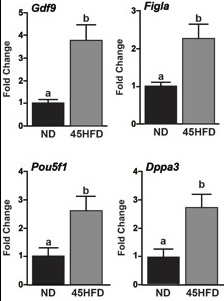
The objectives of these studies are to determine mechanisms by which inflammation and oxidative stress associated with obesity alters mRNA transcription and stability during oocyte development and maturation and how changes in levels of specific mRNAs reduce oocyte competence for embryonic development. Using mouse models of obesity, we have demonstrated increases in candidate mRNAs including Pou5f1, Dppa3, and Gdf9 stored in the ovulated oocyte.
In vitro studies indicate that exposure to oxidative stress during oocyte maturation similarly increases Dppa3 and Pou5f1 mRNAs and increases translation of DPPA3 and POU5F1 protein during embryo development.
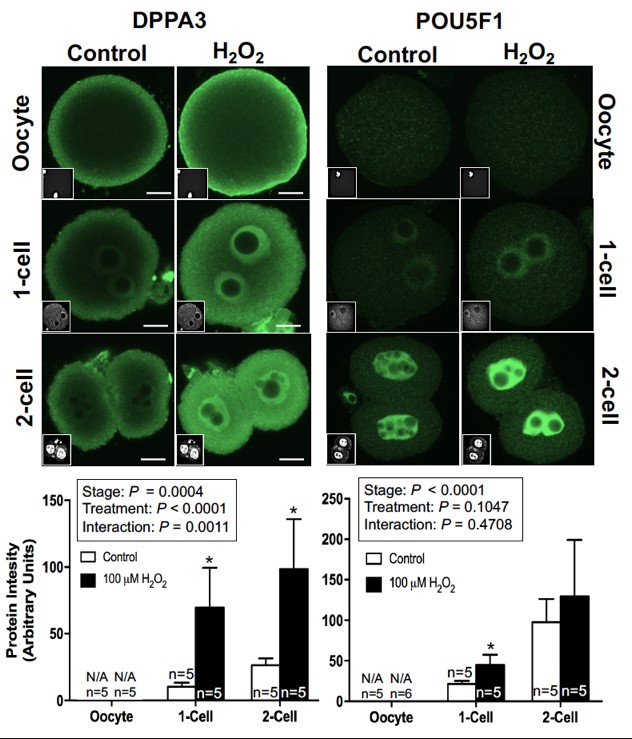
Finally, we have demonstrated a positive correlation between diet-induced changes in gut microbial populations, ovarian inflammation, and oocyte gene expression.
Current studies are discerning transcriptional and post-transcriptional mechanisms that cause the increased abundance of Dppa3 and Pou5f1 in oocytes from obese mice. Furthermore, the impact of these increased mRNAs and proteins on embryo development rate, cell differentiation, and implantation is also under study.
Using a cow model of metabolic stress, we are beginning trials to determine how the gut microbiome changes during heifer attainment of puberty as well as in ovulatory versus anovulatory cows. Molecular and cellular studies are also underway to understand mechanisms of excess androgen synthesis in somatic cells of the ovary in our cow model of metabolic stress.
We will use these collective data to better understand obesity- and chronic stress-dependent infertility in female animals and women in order to overcome early embryonic loss and correct negative adaptive programming associated with poor gamete quality.
Programming
Impact of Parental Obesity on Fetal Programming:
Objectives of these studies are to identify mechanisms by which parental obesity impacts placental function and fetal growth during gestation.  Furthermore, the impact of fetal exposure on metabolism and gut microbiome composition during the neonatal and adolescent periods is also being studied. These studies are being performed using two different mouse models of obesity; one is a diet-induced model and the second is a genetic model of impaired satiety.
Furthermore, the impact of fetal exposure on metabolism and gut microbiome composition during the neonatal and adolescent periods is also being studied. These studies are being performed using two different mouse models of obesity; one is a diet-induced model and the second is a genetic model of impaired satiety.
Our most recent studies show differences in the maternal lipid profile, fetal growth, and placental size dependent on how obesity develops. Preliminary RNA sequencing data from placenta indicate that lipid metabolism and prostaglandin signaling are altered as a result of maternal obesity. There was also a significant impact of paternal obesity on placental gene expression suggesting that reduced gamete quality impacts how the placenta develops and functions. Together these studies suggest complexity regarding how "obesity" during pregnancy impacts placental function and fetal development
Current studies are also being conducted to determine how parental obesity impacts skeletal muscle metabolism, liver and pancreatic function, and establishment of the gut microbiome in viable offspring. Data from these collective models will enable discrimination between mechanisms that lead to negative adaptive programming of offspring metabolism.
Dr. Wood's People

Associate Professor
A224k Animal Science Building
Phone: (402) 472-6437
E-mail: jwood5@unl.edu
Post -Doc Students

Alex Snider
Alex received her BS in Animal Science from Oregon State University in 2013, she then continued her education and completed her PhD in Reproductive Physiology at Oregon State University in 2017. The title of her dissertation was “Effects of Feeding OmniGen-AF® on Superovulatory Response in Donor Beef Cows”. After completion of her PhD, she started a post-doctoral position in January 2018 with a focus of investigating the effects of inflammation on the ovarian microenvironment.
Graduate Students

Katie Bidne
PhD - Animal Science
Dissertation: Impact of Parental Obesity on Key Milestones during Pre-Implanation Embryo Development

Andrea McCain
MS-Animal ScienceDissertation: Maternal obesity alters fetal development of skeletal muscle and neural tissue due to impaired placental function and has lasting effects on adult offspring

Kelsey Timme
MS-Animal ScienceDissertation: Effect of obesity-induced inflammation on regulation of Dppa3 and Pou5fl mRNAs in growing and maturing oocytes

Kerri Bochantin
MS-Animal Science

Katelyn Malone
MS-Animal Science
Undergraduate Students

No current students
Woods Lab Alumni

Dissertation
Meetings
MeetingsFun in the Lab


